It’s Time for Change.
Improved bladder control from urge urinary incontinence (UUI) symptoms1 may be within reach.
When finding the right therapy seems impossible, what’s next? The Altaviva™ implant is your convenient and hopeful next step, with automatic therapy delivery that integrates seamlessly into your life—designed to keep you moving forward, wherever your journey leads.

Trusted Innovation in Bladder Control
Medtronic is the global leader in neuromodulation for bladder and bowel control — the technology used in the Altaviva™ implant — and in lifesaving devices such as cardiac pacemakers. Medtronic pioneered the first neuromodulation bladder device over 25 years ago and continues to innovate, delivering options that may help improve your quality of life.1
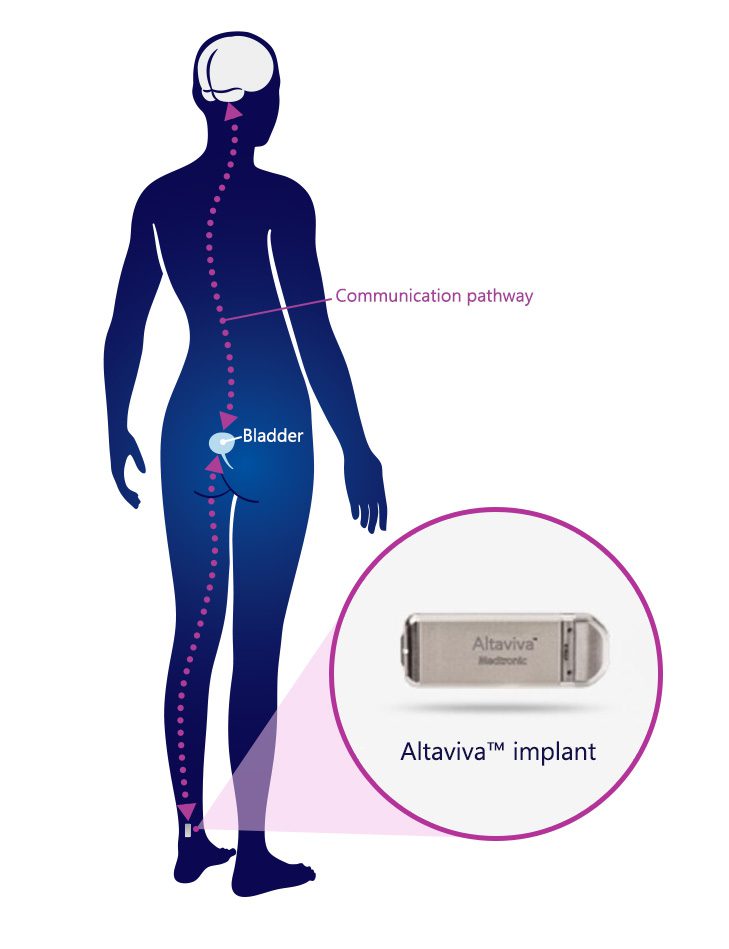
A single procedure may help improve your quality of life:1
- The Altaviva™ implant is placed near your ankle with a minimally invasive procedure2 that does not require general anesthesia or sedation.3
- Same-day therapy activation.3
- MRI-ready from the start for full-body 1.5T or 3T scans.†,4
- Long-lasting battery expected to deliver 15 years of therapy.‡,5
In addition to risks related to surgery, complications can include pain at the implant site, lower leg pain, infection, technical or device problems, movement of the implant, undesirable change in bowel or bladder function, or uncomfortable or unintended stimulation sensations. Please see Important Safety Information for more details. Talk with your doctor about ways to minimize these risks.
Is the Altaviva™ Implant Right for You?
Who should try it?
Everyone experiences symptoms differently, and there are many ways to manage UUI.2 If you have tried other treatments without success, such as medications, ask us today about the Altaviva™ implant.
Take the Next Step Explore the Altaviva™ implant

Consultation
Meet your specialist and review symptoms.

Implant
A small device is placed under the skin near the ankle and sends electrical pulses to the tibial nerve that is important in maintaining normal bladder function.6,7

Imagine the places you’d go if you didn’t have to “GO”
Relief from UUI Symptoms may be possible
Let’s talk about whether the Altaviva™ implant is right for you.
References:
- Appendix B: Clinical Study Summary. M028929C001 RevC – Clinician Therapy and Programming Guide
Altaviva™ Model P7850N.
- Cameron AP, Chung DE, Dielubanza EJ et al. The AUA/SUFU guideline on the diagnosis and treatment of
idiopathic overactive bladder (2024). J Urol. 2024;212:11-20
- M028930C001 RevC – Altaviva™ Model P7850N Neurostimulator Implant Manual
- M028949C001 RevC – MRI Guidelines for Altaviva™ Neurostimulator
- M028929C001 RevC – Clinician Therapy and Programming Guide Altaviva™ Model P7850
- Li X, Li X, Liao L. Mechanism of action of tibial nerve stimulation in the treatment of lower urinary tract
dysfunction. Neuromod. 2023;27:256–266.
- Bhide AA, Tailor V, Fernando R, Vik K, Digesu GA. Posterior tibial nerve stimulation for overactive bladder –
techniques and ekcacy. Int Urogynecol J. 2020;31:865–70.
Important Safety Information:
Medtronic Altaviva™ tibial neuromodulation system treats urge urinary incontinence (leakage). It should be used after you have tried other treatments such as medications and behavioral therapy, and they have not worked or you could not tolerate them.
This therapy is not for everyone. The Altaviva™ system is contraindicated (not allowed) for patients who are poor surgical candidates including patients with open wounds, sores, or damaged skin near the treatment area; current or recent history of poor blood circulation in the legs or open sores on the legs from circulation problems; physical changes or previous surgeries where the Altaviva™ device is placed. You must be able to operate or receive assistance in operating the system to be a candidate.
This therapy is not intended for patients who: are not good candidates for surgery or have conditions that make it hard to heal from wounds (such as uncontrolled diabetes, swelling in the lower leg, or nerve problems in the leg); have metal implanted within 5 cm of where the Altaviva™ device would be placed; have a current or unresolved blockage in the urinary tract caused by things like an enlarged prostate, cancer, or urethral narrowing; are allergic to any materials in the Altaviva™ device. The Altaviva™ system may affect or be affected by other implanted medical devices, including pacemakers and defibrillators.
Talk to your doctor if you have a pacemaker or other implanted devices. You cannot have diathermy (deep heat treatment using shortwave or microwave electromagnetic energy) if you have an Altaviva™ device. Do not place the charger or ankle band on broken or unhealed skin. Safety and effectiveness have not been established for pregnancy; patients under the age of 18; patients with progressive, systemic neurologic disease; patients with history of urinary retention, or bilateral stimulation. In addition to risks related to surgery, complications can include pain at the implant site or lower leg pain, infection, wound complications, nerve injury, movement of the implant, undesirable change in bowel or bladder function, uncomfortable or unintended stimulation sensations, unexpected shocking sensation, loss of therapeutic effect, discomfort when recharging, or technical or device problems.
This therapy is not for everyone. This treatment is prescribed by your doctor. Please talk to your doctor to decide whether this therapy is right for you. Your doctor should discuss all potential benefits and risks with you. Although many patients may benefit from the use of this treatment, results may vary. For complete safety information about this treatment, please visit the Medtronic website at www.medtronic.com. USA Rx Only. Rev 0925
©2025 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. US-UP-2500701
